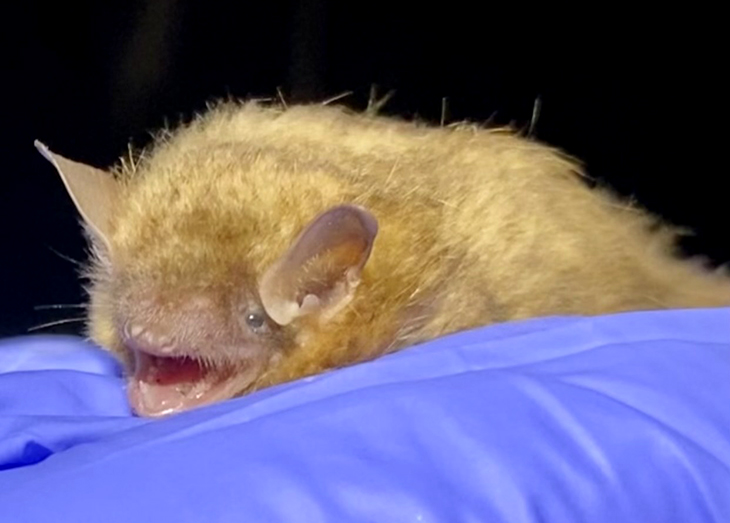
USFWS-funded project helps track endangered Ohio bats
White-nose syndrome is wiping out Ohio’s tri-colored bat populations, and researchers are racing to understand more about[…]

Research aims to tackle problems measuring biodiversity change
We’ve all heard about dwindling biodiversity across the planet, but it turns out scientists have a difficult[…]

Scientists program proteins to pair exactly
While the Ohio State Buckeyes and Washington Huskies prepared to butt heads in the Rose Bowl on[…]

Machine learning helps predict endangered plant species
There are many organizations monitoring endangered species such as elephants and tigers, but what about the millions[…]

A close-up look at the whirlpool around gigantic black hole
Imagine if you could see something the size of a coin at the distance of the moon.[…]

Ohio State team helps map the Arctic like never before
Some portions of the Arctic are so remote and barren, they seem like another planet. In fact,[…]
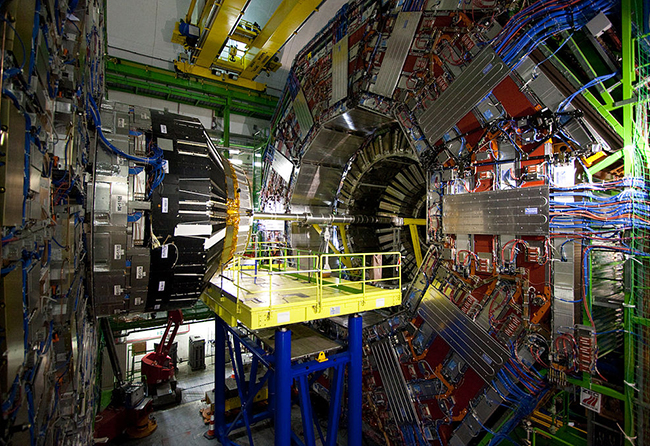
Physicists uncover new behavior of elusive Higgs boson particle
It’s been six years since scientists confirmed the existence of a long-theorized particle called the Higgs boson,[…]

Venom researchers making powerful discoveries
For wild animals, life is all about survival. And most don’t have the luxury of cheetah-fast speed[…]